EN-374 is an investigational gene therapy that works by adding a functioning copy of the CYBB gene directly into the body. It is intended to restore critical immune function for people living with X-CGD. The gene therapy uses a new in vivo approach that does not involve a stem cell transplant, apheresis, or myeloablative conditioning.
All participants in the Ennovate trial will receive EN-374.
There is no placebo in this study.
What you need to know about the Ennovate CGD clinical trial
- The medication being studied is a gene therapy intended to restore critical immune function for people living with X-CGD.
- The gene therapy uses a new in vivo approach that does not involve a stem cell transplant, apheresis, or myeloablative conditioning.
- You will be treated by a medical team with experience in CGD.
- All study visits, including travel and lodging, are provided at no cost. You will also receive a stipend for taking part in the study.
- You will be helping study the safety and effectiveness of a treatment for people living with X-linked CGD.
Eligibility
Male
Diagnosed with X-linked CGD
Have not received a
bone marrow transplant
NOTE: UK participants must be 18 years of age of older and have an EN-374 capsid total antibody titer ≤ 1:512.
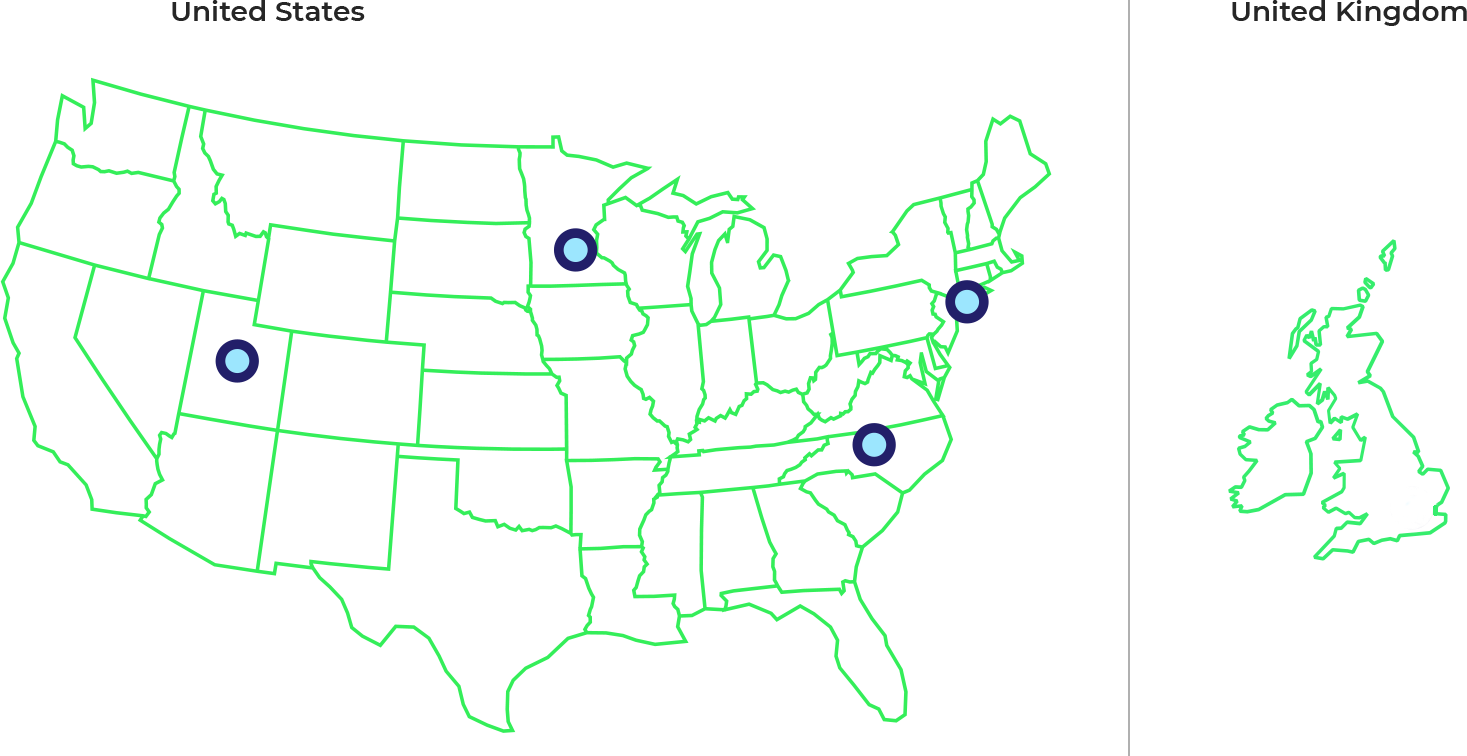
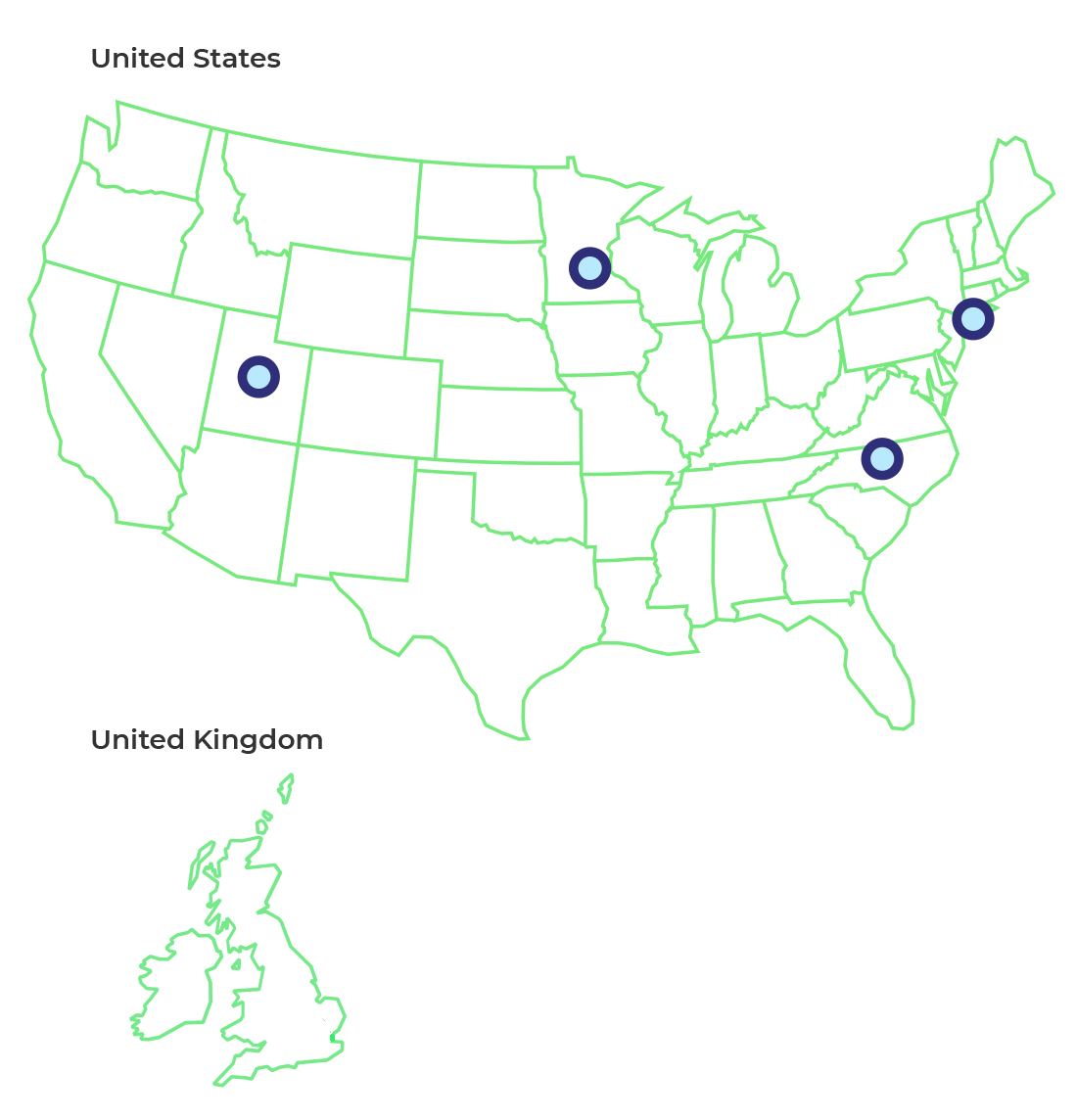
Clinical Trial Study Locations
Multiple clinical trial sites are being opened across the US. If you are interested in learning more or participating, please speak with your physician or reach out to one of the investigators listed below and on clnicaltrials.gov, study identifier
NCT06876363.
All Travel Expenses and Lodging Reimbursement Included for the Participant + One Caregiver
Please contact the nearest study center below for additional information.
New York
Columbia University
Irving Medical Center
Morgan Stanley Children's Hospital
New York, NY 10034
Contact:
Brianna Mayo, PNP, RN
212-305-2372
Principal Investigator:
Joseph Oved, MD
Minnesota
University of Minnesota
Minneapolis, Minnesota 55454
Contact:
Alli Travis, BSN, RN, BMTCN
612-625-6150
Principal Investigator:
Christen Ebens, MD, MPH
North Carolina
Duke University
Durham, North Carolina 27710
Contact:
Linda Brown
919-613-1482
Principal Investigator:
Talal Mousallem, MD
Principal Investigator:
Kris Mahadeo, MD, MPH
Utah
University of Utah, Primary Children's Hospital
Salt Lake City, Utah 84113
Contact:
Morgan Badgley, BS, CCRC
801-662-4813
Principal Investigator:
Ahmad Rayes, MD, MSc
*Additional locations opening soon
FAQ's
What is a clinical trial?
A clinical trial is a research study conducted with patients and designed to evaluate the safety and effectiveness of a new treatment. A trial helps determine whether a new therapy is safe and works as intended.
Will I be compensated for participation in the trial?
The trial sponsor will cover transportation and lodging costs associated with study visits for the participant and a caregiver. A daily stipend will be provided for each study visit completed. Please contact the nearest study center for additional information.
What if I don’t see a location near me?
Please contact the location that is closest to you for more study information and travel options. Reimbursement for travel and lodging is available for the participant and a caregiver.
Who is conducting the trial?
The sponsor of this clinical trial is Ensoma, a biotechnology company whose mission is to develop potentially curative medicines for genetic diseases, immune disorders, and cancer through in vivo hematopoietic stem cell engineering.